Update: 22 November 2024
Water
Author: Julie Casper, C. Ac.
Water is Life. Water is essential for life. It predates life and is a prerequisite for it. Cells in all living organisms consist of 70% water, making it the predominant component of life. Nutritionally, it is the essential nutrient. What is unique about water is that it is the only substance that can exist in all four states of matter: liquid, gel, solid, and gas. It is both finite and infinite.
Science has recently discovered that water incorporates memory. There is communication between distinct units. This is a new paradigm that challenges what we THINK we know about ...everything. Water remains a scientific and metaphysical mystery.
Contents
- Drinking Water (how-to)
- What water is best to drink?
- Don't Drink The Water
- Water mineral content and its affect on health.
- Arsenic Toxicity
- Hydraulic Fracturing (Fracking) - Effect on Drinking Water
- Basic Drinking Water Options
- How to Make Your Drinking Water Safe
- Demineralized Water
- How to Remineralize Water
- Recommended Drinking Water
- Water Filtration
- Water Softeners
- Water Information Sources
- Resources

Water and oxygen are the two most essential substances to life. A water molecule consists of two hydrogen atoms bonded to a single oxygen atom "H2O."
Drinking Water (how-to)
Water is an essential nutrient. Next to oxygen, water is the most important factor for survival. It is more important to have an adequate intake of water than it is to have enough calories. While the amount of water in each person varies, the average adult is 60% to 70% water (about 10 to 12 gallons). To maintain an adequate supply of water for essential biological functions, the average adult should drink 8 to 10 glasses a day.
Water is responsible for many essential metabolic processes, including delivering nutrients and oxygen to cells, cushioning joints, hydrating skin, converting food into energy, removing toxins and wastes, and supporting the body's natural healing processes. How much water you need to drink depends on several variables. If you're thirsty, you are probably slightly dehydrated already. As we age our bodies are less able to sense dehydration and send thirst signals to the brain, so if you are over 50 don't rely on thirst alone. When you are active, you lose more water than when you are sedentary. Diuretics such as caffeine (coffee, tea and soda), alcohol and even some prescription medications cause you to excrete more water than you normally would. Avoid diuretics when possible because over time your body will regulate itself to that diuretic effect. In addition to urination, you also loose water through respiration, perspiration and bowel movements.
Back to Top
The Water Prescription, by Christopher Vassey is a guide to how water can prevent and treat disease and rejuvenate the body and mind. Learn how water deficiency impacts many diseases and other health disorders. It explains how to determine the quality and quantity of water that is best for you and the time during the day it is best to drink. Dr Vassey includes 10 water cures for profound physical rehydration, toxin removal, and remineralization.
How do you know if you're drinking enough water?
To estimate how much water you should drink, take your body weight in pounds and divide that number in half. This is the volume in ounces of water a day that you need. For example, if you weigh 150 pounds, you should drink at least 75 ounces of water a day. With exercise, add another eight ounce glass of water for every 20 minutes you are active. If you live in an arid climate you need to drink more. Flying in an airplane dehydrates you too, it's a good idea to drink eight ounces for every hour you're on board the plane. To prevent dehydration, for every caffeinated or alcoholic beverage you should drink an equivalent amount of water.
Dehydration (hypohydration)
Dehydration is surprisingly common because most people do not drink enough water and overuse diuretics. When there is not enough body water, a disruption of metabolic processes occurs. The term dehydration usually refers to hypernatremia (loss of free water and the attendant excess concentration of salt). Dehydration occurs when water loss exceeds water intake, usually due to nutrition, exercise or disease.
Effects of Decreases in Body Water Levels (Dehydration)
- Most people can tolerate a 3% - 4% decrease in body water.
- A 5% - 8% decrease may cause fatigue and dizziness.
- A decrease of over 10% can cause physical and mental deterioration, accompanied by severe thirst.
- A decrease more than 15% - 25% of the body water is invariably fatal.
Symptoms of Dehydration
Dehydration symptoms generally become noticeable after 2% of normal euhydration water volume has been lost. In people over age 50, the body's thirst sensation diminishes and continues diminishing with age. Many senior citizens suffer symptoms of dehydration. Dehydration along with hyperthermia results in the elderly dying suddenly during extreme hot weather.
| Common Symptoms of Dehydration | Symptoms of Chronic Dehydration |
|---|---|
| Thirst | Abnormally Dark Urine |
| Headache | Constipation |
| General Discomfort | Loss of Skin Elasticity |
| Loss of Appetite | Decreased Blood Pressure (hypotension) |
| Dry Skin | Dizziness or Fainting Standing Up (due to orthostatic hypotension) |
| Decreased Urine (volume) | Rapid Breathing |
| Confusion | Listlessness |
| Unexplained Tiredness | Insomnia |
Notes: Athletes may suffer a loss of performance, experience flushing, low endurance, rapid heart rates, elevated body temperatures, and rapid onset of fatigue. Untreated dehydration generally results in delirium, extreme lethargy, seizures, sunken fontanelle ‘soft spot’ of the skull in infants, fainting, sunken eyes, unconsciousness, swelling of the tongue, and in extreme cases death. A blood test may show hyperalbuminemia (overabundance of protein in the serum), poor kidney function or excess concentration hemoglobin. Urinalysis may show concentrated urine.
Back to TopWater is Life … and the quality of water determines the quality of life. Lake Superior Binational Forum
What water is best to drink?
Once upon a time, safe, mineral-rich drinking water was easy to find and free. Water is so essential, it is important to understand your drinking water options. And it's not that complicated.
- Either you find a pure natural (unprocessed) drinking water source,
- Or, you need to decontaminate your drinking water — somehow.
Decontaminating the water you drink is where choice can get confusing. There are so many products and services competing for your money. Tragically, there is no perfect solution.
Everyone's filtering needs are different based on their water's specific contaminants. You first need to test your water to discover what these are, and water tests can never be completely accurate. There are professional testing services and DIY test kits. You might want to start with DIY, especially if you suspect a specific contaminant that you can check for with the kit. Testing for everything is cost prohibitive, if not impossible.
If you are thinking that your tap water should be okay because "wastewater treatment plants" solve the problem, think again. Because even the very best managed, most technologically advanced water treatment technologies cannot begin to address the sheer volume and variations of chemical toxicants flowing into U.S. water supplies today.
Back to TopDon't Drink The Water

‘Don't Drink the Water’
The U.S. government currently has a Chemicals in Commerce list with more than 85,000 chemicals on it. There are approximately 2,500 "high production volume" (HPV) chemicals, which are manufactured at a rate of more than one million pounds annually. That's 90 pounds per person, per day. Nearly 45 percent of these HPV chemicals lack adequate toxicological studies needed to evaluate their health effects on humans and wildlife. And an unknown number of new chemicals are not among this total.
What's more, only new organic chemicals are added to the ‘chemicals in commerce’ list. Organic in this instance does not mean natural wholesome goodness, it is simply referring to a class of chemicals which contain carbon. What's worse, the new chemicals that are exempt from the official listing process include inorganics, pesticides, food additives, some large polymer molecules and any chemical produced in low quantities!
Only a handful of synthetic chemicals have even been tested for human health impacts — and NONE have been tested for combined effects, or synergistic health impacts. In the U.S., industry admits to releasing over 4 billion pounds of toxic chemicals a year. About 2,000+ new chemicals are introduced annually in the U.S. alone. Some of these chemicals act as endocrine disrupters, disrupting normal hormone function, and can produce effects at the parts per billion or parts per trillion level.
20 percent of the groundwater that Californians rely on carries high concentrations of contaminants like arsenic, uranium, and nitrate. Tom Philpott, Mother Jones
The effects of some ECCs (emerging chemicals of concern) are trans generational - this means that when animals are exposed in utero, effects are transmitted not only to the offspring, but are inherited for generations thereafter, often compounding concentrations. In addition, scientists are worried about the effects from exposures to mixtures of these ECCs and/or other chemicals.
10 trillion pounds of chemicals are produced per year in the US. That equals 90 pounds per person PER DAY! 85,000 chemicals are listed on the Toxic Substances Control Act. Richard Denison, PhD, Lead Senior Scientist, Johns Hopkins School of Public Health
A notable source of chronic antibiotic exposure is our water. Modern agriculture has introduced GMOs into the food supply. GMO crops are reliant on glyphosate, a potent antibiotic (biocide). The agricultural runoff of glyphosate, 2-4D, medical antibiotics in the manure spread on fields (as much as 97% unmetabolized), and the unmetabolized antibiotics from human excretion, ultimately end up back in the water supply. Water treatment facilities are not equipped to remove these types of synthetic chemical compounds. The EPA standards for glyphosate in water in America is .7ppm. European research demonstrated organ damage to animals at .1ppb (.0001ppm) of glyphosate in water. U.S. water levels allow glyphosate at levels 7,000 times higher than what has been shown to be toxic in animals.
Under a 20-year-old water recycling program, wastewater that is generated as a by-product from oil extraction is treated and sold to some 90 Southern California landowners — including one with certified organic operations — which use it to grow crops such as citrus, almonds, apples, peaches, grapes, and blueberries sold in major grocery chains around the country. Source: Josh Harkin Mother JonesBack to Top
Water mineral content and its affect on health.
Paraphrased from a TEI News Bulletin ©1986, by David Watts, Director of Research Trace Elements Laboratory.

The U.S. Environmental Protection Agency (EPA) has established drinking water standards for nutrient mineral and toxic metal content. However, detrimental effects have been observed and documented even when mineral levels occur well within EPA ‘acceptable ranges.’ Organic contaminants, such as chlorinated hydrocarbons, insecticides, or toxic heavy metals always have harmful health consequences, although to different degrees in different individuals.
Depending on the susceptibility of an individual, the nutritional mineral dominance, or relative ratio imbalances in the water supply also can have a significant impact on their health. Some people appear to be more susceptible than others to toxic exposure via water. The reason for this can be better understood by determining their distinct metabolic characteristics, including;
- nutrient mineral status,
- metabolic utilization,
- absorption capabilities and retention rates, in relation to the mineral content in their water.
Soft Water
Soft water is characterized as being acidic (pH less than 7.0), with total hardness in the range of less than 180 mg/L. Often, high sodium levels, or high sodium in relationship to other specific minerals, are found occurring naturally in soft water.
Soft water = hard arteries. The relationship of soft water influencing the incidence of cardiovascular disease death rates was first documented in 1957 [Kobayashi 1957]. Since then, studies have confirmed that in areas with soft water, compared to hard water regions, death rates from cardiovascular disease were significantly higher. Sodium may be a main contributor to various health pathologies found in soft water regions.
- Mortality and Hardness of Local Water Supplies
- Nutrients in Drinking Water World Health Organization
The process of artificially softening water will also result in elevated sodium levels relative to calcium and magnesium. Treated or softened water usually does not raise the sodium content above acceptable ranges, however, the sodium concentration can be over one-thousand times higher in relation to calcium and magnesium levels. Susceptible individuals, or those who have a tendency to retain sodium, would be adversely affected by consuming water with high concentrations of sodium (real or ratio-wise).
People who drink soft water that is delivered via copper water pipes are likely to have symptoms of copper toxicity. Because soft water has been demineralized, it absorbs the minerals that it comes into contact with. If your water is running through copper pipes, it will absorb copper. If you suspect you have copper toxicity, you can download a Copper Toxicity Self-Evaluation checklist here:
Hard Water
"Hard water" is usually characterized as having a pH greater than 7.0, with total hardness in the range of 250 mg/L or greater. Since most adverse health affects appear to be contributed to by soft water, it would seem logical that hard water is ideal for everyone. However, hard water can also contribute to mineral deficiencies, or imbalances. As an example, excess calcium can antagonize the absorption of other minerals, such as iron, zinc and potassium. Studies indicate that zinc can be negatively affected by the consumption of hard water compared to soft water.
Health effects of specific minerals found in water.
- Calcium and Magnesium
-
The minerals calcium and magnesium are known to prevent increased sodium accumulation within the body. Intake of water containing excess sodium however, can contribute to calcium and/or magnesium deficiencies systemically. This is especially true if dietary calcium or magnesium intake is marginal or inadequate.
Calcium produces a stimulatory or hyper-excitable effect upon muscle tissue, while magnesium produces a sedative effect. A magnesium deficiency relative to calcium will produce vasoconstriction. Magnesium has been generally accepted as being the protective factor in preventing cardiovascular disturbances, due to the beneficial effects of magnesium when administered during the treatment of cardiovascular disorders. Heggvelt and coworkers reported that magnesium levels in infarcted myocardial tissues averaged 42 percent less than in non-infarcted cardiac tissue. Other studies have also reported that levels of magnesium are higher in the cardiac tissue of individuals who had lived in hard water areas and who had died of accidental causes as compared to heart attack victims.
- Zinc-to-copper balance and ischemic heart disease (IHD)
-
The zinc-to-copper relationship should also be scrutinized, in both hard and soft water. Evidence presented by Dr. Leslie Klevay has shown that a high serum zinc-to-copper ratio, or a relative copper deficiency may contribute to IHD. A deficiency of copper relative to zinc can cause a decrease in HDL (high density lipoproteins). An elevated tissue zinc-to-copper ratio (17 to 1 or greater) would indicate susceptibility if water with this same mineral configuration is being consumed.
A low zinc to copper tissue ratio can also contribute to atheromatous development. Excess tissue copper accumulation is known to have a suppressing effect upon the thyroid gland. Hypothyroidism has long been associated with elevated serum cholesterol. Excess copper is frequently found in soft water, due to the corrosive effect of soft water acidity upon copper pipes. If substantial amounts of copper are being leached from the pipes, it is readily evident by the deposit of copper sulfate in the sinks. A bluish green discoloration will develop if the water drips from the faucet overnight.
- Chlorine and ischemic heart disease (IHD)
-
The chlorine content of water may also be a factor to consider in heart disease. Studies reported by the E.P.A. (using pigeons as model), found that cholesterol levels increased in the group drinking chlorinated water while on a low calcium diet. This group exhibited a fifty percent increase in cholesterol over the control group. Calcium intake was found to prevent this rise in cholesterol, even when chlorine and fat were present in the diet. Chlorine is used as a disinfectant in drinking water and may range from one to three milligrams per liter. Even though the water in the animal studies contained ten milligrams per liter, a susceptible individual may be affected by chlorine in much smaller amounts.
The studies are continuing, and at present they indicate that hard water (high calcium and magnesium), as well as a diet adequate in calcium may hinder atherosclerosis when chlorine is present.
These studies would also suggest that patients suffering from IHD avoid chlorinated water. Individuals with health problems prone to atheromatous formation, such as hypothyroidism, diabetes, hyper cholesterol and hypertriglyceridemia should also be cautioned.
Recent studies have also shown evidence that links chlorinated water supplies to an increased incidence of colon, rectal and bladder cancer. This is apparently due to the interaction of chlorine with other chemicals in the water, producing carcinogens.
Health effects of toxic metals found in water.
Mineral assays of soft water will frequently reveal concentrations of toxic metals above E.P.A. acceptable levels. These include; copper, lead, manganese and cadmium. The high concentration of toxic metals is usually due to the corrosion of water pipes, soldered joints and faucets used in the home. Increased amounts of toxic metals in water contributes to an increase in various adverse health effects associated with these metals, children and the elderly are especially susceptible.
- Lead
-
Lead content in water will tend to be higher in areas of soft water and lower in hard water areas. Lead concentrations of water in the Boston area, reported in a 1981 study, were found to be above E.P.A. standards in fifteen percent of the homes tested. The average pH of Boston water ranged between 6.0 and 7.0 with a hardness of 14 mg/L, as compared to water tested in the Columbus, Ohio area, in which the pH was 9.6 with a hardness of 101 mg/L.
It has been shown that lead concentrations can be dependent upon how long the water has been standing in contact with the pipes, soldered joints and faucets. Lead concentrations will increase as the duration of time the water is exposed to pipes and joints increases. Normally, concentrations will decrease after a five-minute flush, and will again reach these previous levels by the mid-afternoon. This reflects the effect to which soft water leaching may have upon increased toxic metal tissue concentrations in individuals, such as with the heavy metal lead.
- Cadmium
- Cadmium has been implicated in cardiovascular disease and renal dysfunction. Dr. Henry Schroeder found that cadmium in the drinking water was the most effective of the heavy metals in producing hypertension, heart enlargement, atherosclerosis and kidney disease in animals. These manifestations resembled those problems seen in humans. Cadmium has a tendency to accumulate in the kidneys, arteries and liver. It also competes with the mineral zinc, resulting in an interference with zinc related enzyme functions. Dr. Schroeder stated that the zinc-to-cadmium ratio naturally occurring in the earth's crust is between 500 and 1000 to 1. We have established the ideal zinc to cadmium tissue levels at 500/1. At this ratio or higher, zinc is protective to the adverse affects of cadmium. Drinking water should have at least a 300/1 ratio of zinc-to-cadmium to be considered safe for consumption.
Metabolic disorders associated with soft and hard drinking water.
- Osteoporosis
- Prolonged intake of soft water can contribute to osteoporosis, especially in individuals with a predisposing systemic mineral pattern. Water containing inadequate amounts of magnesium relative to calcium has been shown to be related to a greater incidence of bone fractures with decreased healing time of bones in the elderly. Magnesium deficient soils and water have also been related to osteoporosis and dental caries, even while the calcium content is adequate or high. It is recognized that a high calcium to magnesium ratio results in decreased bone matrix formation.
- Hypertension and Arthritis
- Drinking water that contains high amounts of iron can lead to excess tissue deposition. The primary deposition sites are in the liver, joints and muscle. Excess accumulation is associated with arthritis, cirrhosis and hypertension.
- Hyperactivity (ADD, ADHD, ODD, et al.)
- Elevated lead accumulation has been well documented in contributing to hyperactivity in children, as well as other intellectual deficit disorders. Children are very susceptible to lead retention. This has been seen consistently in tissue mineral patterns, in which the majority of children show low calcium tissue levels, or low tissue calcium-to-lead concentrations. Calcium intake in adequate amounts is necessary to protect from the highly detrimental effects of lead and its absorption. When calcium intake is below ideal, lead is readily absorbed and will be deposited similarly and in place of calcium.
- Premenstrual Syndrome (PMS)
- With the increased use of copper pipes and soft water systems in homes today, excess copper accumulation in the tissues is now found more frequently in a greater percentage of the population. Perhaps the most susceptible population group is women. Copper toxicity or a relative zinc deficiency has been found in women suffering from PMS and emotional disorders. Elevated tissue copper accumulation contributed to by oral contraceptive agents and copper IUD's, and further added to from water sources can contribute to a significant copper burden.
- Kidney Stones
- Soft water areas of the United States have also been correlated with an increased incidence of renal calculus formation. This condition is related to a deficiency of magnesium relative to calcium. The increased consumption of soft water during the summer months, and increased vitamin D synthesis due to exposure to sunlight, increases the amount of calcium absorption resulting in a relative magnesium deficiency. This may explain the seasonal occurrence of kidney stone formation during the summer months.
General hTMA protocol recommendations for drinking hard or soft water.
- Individuals who have hTMA patterns which reveal low tissue calcium and magnesium levels in relation to high sodium and potassium, should avoid soft water (or water that has been artificially softened) especially if the patient is subject to hypertension, or ischemic heart disease (IHD). Hard water would be considered therapeutic or protective for these individuals.
- Individuals who have hTMA patterns which reveal high levels of calcium and magnesium levels, relative to sodium and potassium may find therapeutic benefit from the use of soft water.
Whether or not the originating source of the water is from a municipal water supply or a private water supply, drinking water often becomes contaminated after it reaches the home plumbing system. Acidic water and deteriorating home plumbing results in heavy metals leaching from pipes. As a result, the excessive levels of some metals may have a negative effect on health, or may also contribute to further elevation or imbalances of levels already found within the body.
Mineral analysis (hTMA), combined with an elemental water chemistry report can be applied in clinical practice to expose how household water may be affecting hTMA protocol results. If a patient is not responding favorably to therapy (for seemingly unknown reasons), their drinking water may be responsible. If this is the case, corrective minerals may then be added to the water.
Back to TopArsenic concentration in new wells in Minnesota from August 2008 – July 2013
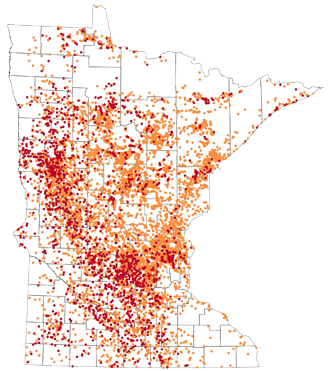
- Arsenic concentrations greater than 2 µg/L but less than 10 µg/L (orange dots)
- Arsenic concentrations rations greater than 10 µg/L (red dots)
Two micrograms per liter (µg/L) is the lower detection limit for arsenic used by most laboratories. Ten micrograms per liter µg/L is the Maximum Contaminant Level (MCL) for arsenic, and is the national enforceable standard for community water supplies. Source: Minnesota Department of Health - Well Owner's Handbook
Arsenic Toxicity
Arsenic is public enemy number one on ATSDR's Priority List of Hazardous Substances list. Arsenic is a known carcinogen and affects the skin, digestive system, liver, nervous system and respiratory system. Arsenic compounds can create reactions in the body that disrupt enzymes that are involved in respiration of cells, fat and carbohydrate breakdown and their metabolism. The accumulation of toxic levels of arsenic can result in paralysis, coma, cardiovascular collapse and death.
Today in the United States, the quantity of arsenic released by human activities exceeds amounts released from natural sources by at least threefold. The major sources of arsenic release to the environment are;
- Coal fired power plants.
- Arsenic-treated lumber.
In modern industrial agriculture (e.g., CAFO's) organic forms of arsenic are actually fed to pigs and poultry to improve production. And in the case of swine, to treat diarrhea. This meat is then used in commercial food products. Also, arsenic can be found in many commonly used products including fungicides, pesticides, herbicides, laundry products, cigarette smoke, paints, and wood preservatives. Global industries such as mining and smelting, chemical and glass manufacturing produce arsenic as a by-product. This in turn finds its way into our water supplies and food sources.
High arsenic levels in hTMA results
Clinically, we are seeing more individuals showing elevated levels of arsenic in their hTMA test results. Arsenic occurs naturally in the groundwater. For most of human history we drank our water from the planet's surface, but in modern industrialized civilization the most common source of drinking water is from wells (mined water). Many wells produce water which exceeds 10 micrograms per liter (µg/L), the federal limit for drinking water. Long-term consumption of arsenic above the drinking water limit may increase the risk of health problems of the skin, circulatory system, or the nervous system, including some cancers. Every private well should be tested at least once to determine if arsenic is present in the water, and at what levels. Arsenic levels in groundwater will not usually change much over time. Long-term consumption of well water with arsenic levels above 10 µg/L should be avoided.
Back to TopHydraulic Fracturing (Fracking) - Effect on Drinking Water
Human exposure to fracking chemicals can occur by ingesting chemicals that have spilled and entered drinking water sources, through direct skin contact with the chemicals or wastes (e.g., by workers, spill responders or health care professionals), or by breathing in vapors from flowback wastes stored in pits or tanks. If there is a fracking project nearby, your drinking water is at risk. The hydraulic fracturing process injects a toxic fracking fluid deep into the ground at a high pressure in order to fracture shale rock. The cracked shale releases the natural gas that's sequestered inside.
There are 500,000 active gas wells in the U.S. and each gas well requires (on average) 400 tanker trucks to carry water and chemicals to and from the site. It takes 1-8 million gallons of water to complete each fracking job. 72 trillion gallons of water and 360 billion gallons of chemicals are needed to run the wells in operation today. Water is mixed with sand and chemicals to create fracking fluid. Approximately 40,000 gallons of chemicals are used per fracturing. As many as 600 chemicals are used in the fracking fluid mix, known carcinogens and toxins, including;
- Lead
- Uranium
- Mercury
- Ethylene Glycol
- Radium
- Methanol
- Hydrocloric Acid
- Formaldehyde
The fracking chemical cocktail is pressure-pumped deep into the earth's hydrosphere. During this process, methane gas and toxic chemicals leach out and contaminate nearby groundwater. In fact, methane concentrations are 17x higher in drinking-water wells near fracturing sites than in normal wells. Contaminated well water is used for drinking water for nearby cities and towns. There have been over 1,000 documented cases of water contamination next to areas of gas drilling as well as cases of sensory, respiratory, and neurological damage due to ingested contaminated water. The waste fracking fluid is left in open air pits to evaporate, releasing harmful VOC's (volatile organic compounds) into the atmosphere, creating contaminated air, acid rain, and ground level ozone. Hydraulic fracturing yields about 300,000 barrels of natural gas per day. The result includes heavy environmental impacts, numerous safety hazards, and serious health consequences.
- Dangers of Hydraulic Fracturing
- Health hazards in fracking's chemical cocktail
- Hydraulic Fracturing 101
Basic Drinking Water Options
The current state of drinking water is an indictment on our planetary stewardship. If we want to avoid poisoning ourselves (and our children), we must find an uncontaminated water source. For most of us this necessitates some method of water decontamination or "purification." In an attempt to deal with the overwhelming contamination of our drinking water, various processing techniques are being used, from gentle filtration to the increasingly more aggressive refinement such as heat distillation.
Two drinking water options:
- Unprocessed water. For most of human history, our ancestors drank natural (unprocessed) water from surface water including streams, rivers, lakes, and dug wells, and from ground water accessed through springs and artesian wells whose source are aquifers. Aquifers are deep underground pockets containing water that over time, has slowly filtered down beneath the bedrock. In modern industrialized civilization a common source of drinking water is from wells (mined water). This may be one reason for the high arsenic levels we are seeing in clinical hTMA.
- Processed water. Examples of processed water include; tap water from a local water utility, tap water water that is re-filtered at home, bottled water, etc.
Water that comes from a natural spring is unprocessed. It contains a multitude of minerals and gases dissolved into a matrix of H2O molecules. Water that has been filtered using a carbon element is the most commonly used filter and is a gentle form of processing, where some of the water's naturally occurring constituents have been removed. Distilled or reverse osmosis water is aggressively processed water, it's highly refined, essentially chemically pure H2O molecules and is devoid of nature's complex matrix of ingredients.
Distilled water can be used for drinking on occasion. However, distillation does not remove chemicals such as chlorine, trihalomethanes or volatile organic chemicals (VOCs) and pharmaceutical compounds. And because of its chelation effect, long term use is harmful because it will deplete the body of needed essential nutrient minerals.
Back to TopBottled Water
In March 2018, The World Health Organization (WHO) announced it will review potential risks associated with microplastics found in drinking water. The WHO initiative was prompted by research of the most popular bottled water brands which found that more than 90% contained tiny pieces of plastic. A previous study also found high levels of microplastics in tap water.
Coca-Cola® told the BBC it had strict filtration methods, but acknowledged the ubiquity of plastics in the environment meant plastic fibres "may be found at minute levels even in highly treated products."
- Download Report: Microplastics Contamination in Bottled Water
93% of bottled water showed some sign of microplastic contamination. Department of Geology & Environmental Sciences, Fredonia.edu NY
We simply cannot afford the financial, environmental, and health costs associated with drinking bottled water. The next time you go to buy a bottle of water consider the following facts:
- Bottled Water Costs Consumers
- U.S. consumers purchased 8.45 billion gallons of bottled water in 2009. Americans spent $10.6 billion on bottled water in 2009 (1,000 times the cost of tap water). Ironically, half of all bottled water (48.7 percent) came from municipal tap water supplies.
- Bottled Water is Terrible for the Environment
- About 75 percent of the empty plastic bottles end up in our landfills, lakes, streams and oceans. Bottled water wastes fossil fuels in production and transport. Bottled water production in the United States used the energy equivalent of 54 million barrels of oil to produce and transport plastic water bottles in 2007 — that's enough to fuel about 1.5 million cars for a year.
- Bottled Water isn't Safer or Better than Tap water
- Tap water in the United States is subject to more stringent federal safety regulations than bottled water. Federal, state, and local environmental agencies require rigorous testing of tap water safety and make test results available to the public. And despite the marketing claims of purity, independent testing of 10 different brands of bottled water conducted in 2008 found 38 contaminants.

A glass bottle is the best container choice for water. To protect your health, avoid drinking from any plastic bottle (or aluminum can). City tap water must meet standards for some toxic or cancer-causing chemicals, such as phthalate (a chemical that can leach from plastic bottles). However, the industry has managed to persuade the FDA to exempt bottled water from the same regulations regarding these chemicals.
Before you buy bottled water, check the plastic recycle symbol.
A glass bottle is the best container choice for water. Plastic bottles are riddled with problems (mentioned above) and can release toxic heavy metals and chemicals. The triangular shaped numbered recycling symbols stamped on plastic containers and products does not mean the product is recyclable. Not all plastics are recyclable or even reusable.
Within each triangle symbol there is a number (ranging from #1 to #7). This number is important to understand. The purpose of the number is to identify the type of plastic used for the product. There are numerous plastic-based products that do not break down quickly and cannot be recycled. Understanding the plastic codes will make it easier to choose plastics and to know which plastics to recycle. For example, #3 or #5 water bottles are not accepted by most recyclers. A #3 indicates that the water bottle has been made from polyvinyl chloride (PVC, dubbed "poison plastic" because it contains numerous toxins). A #5 means that it's polypropylene (PP) which is considered by some as safe for reuse. The numerous problems associated with plastic seem to outweigh its benefits. Must we continue to be addicted to plastic?
Option - When you have to buy bottled water
In a pinch (e.g. if you are on the road), you can buy a gallon of distilled water (i.e., demineralized water). It's cheaper, less toxic and typically sold in #2 plastic containers. However, distilled water has no minerals and thus acts as a chelator by attracting minerals it comes in contact with in the body. Distilled water may be used for drinking occasionally but is not recommended for drinking for any length of time because of its chelation effect.
The Seven Classifications of Plastics - What They Mean
The plastics industry is legally required to put the appropriate recycle number code on their products. But, it is up to you to understand these codes. Knowing these codes will help you minimize, or avoid, the negative health consequences of exposure to toxic chemicals found in plastics.

- #1 PETE/PET (Polyethylene Terephthalate)
-
PETE/PET are single-use bottles, repeated use increases the risk of leaching and bacterial growth. PETE/PET #1 plastic is difficult to decontaminate, and proper cleaning requires harmful chemicals. Polyethylene terephthalates can leach carcinogens, toxic heavy metals, and chemicals that affect the hormonal balance. PETE/PET is commonly used in many consumer products and packaging, including water and soda bottles. It is intended for single use applications only; repeated use increases chemical leaching and bacterial growth. PETE/PET plastic is very difficult to decontaminate, proper cleaning requires harmful chemicals.
PETE/PET plastic is recyclable and about 25% of PETE/PET bottles in the US today are recycled. The plastic is crushed and then shredded into small flakes which are then reprocessed to make new PETE bottles, or spun into polyester fiber. This recycled fiber is used to make textiles such as fleece garments, carpets, stuffing for pillows and life jackets, and similar products.
Products made of #1 (PETE/PET) plastic should be recycled after one use, do not reuse.
- #2 HDPE (High-Density Polyethylene)
-
High-density polyethylene (HDPE) or polyethylene high-density (PEHD) is a polyethylene thermoplastic made from petroleum. HDPE #2 plastic is thicker, milkier or opaque. It has a high strength-to-density ratio so it is used in the production of plastic bottles (e.g., milk, detergent and motor oil) toys, and some plastic bags. HDPE is considered by some experts as being safer than the other plastics, it is recommend as a better choice when buying bottled water.
HDPE is commonly recycled for secondary use. It is hard-wearing and does not break down as quickly as other plastics when exposed to sunlight or temperature extremes. Recycled HDPE is used to make picnic tables, park benches, plastic lumber, waste bins, bed liners for trucks and other products which require durability and weather-resistance.
Products made of HDPE are reusable and recyclable.
- #3 PVC (Polyvinyl Chloride)
-
PVC is a soft, flexible plastic used to make clear plastic food wrapping, cooking oil bottles, teething rings, toys for children and pets, and packaging for consumer products. It is commonly used as the sheathing material for computer cables, and to make plastic pipes and parts for plumbing. Because PVC is relatively impervious to sunlight and weather, it is used to make window frames, garden hoses, arbors, raised beds and trellises.
PVC is dubbed the "poison plastic" because it contains numerous toxins which it can leach throughout its entire life cycle. Almost all products using PVC require virgin material for their construction; less than 1% of PVC material is recycled.
Products made using PVC plastic are not recyclable. While some PCV products can be repurposed, PVC products should not be reused for applications with food or for children's use.
- #4 LDPE (Low-Density Polyethylene)
-
LDPE is often found in shrink wraps, dry cleaner garment bags, squeezable bottles, and the type of plastic bags used to package bread. The plastic grocery bags used in most stores today are made using LDPE plastic. Some clothing and furniture also uses this type of plastic.
LDPE is considered less toxic than other plastics, and relatively safe for use. It is not commonly recycled, however, although this is changing in many communities today as more recycling programs gear up to handle this material. When recycled, LDPE plastic is used for plastic lumber, landscaping boards, garbage can liners and floor tiles. Products made using recycled LDPE are not as hard or rigid as those made using recycled HDPE plastic.
Products made using LDPE plastic are reusable, but not always recyclable. You need to check with your local collection service to see if they are accepting LDPE plastic items for recycling.
- #5 PP (Polypropylene)
-
Polypropylene plastic is tough and lightweight, and has excellent heat-resistance qualities. It serves as a barrier against moisture, grease and chemicals. When you try to open the thin plastic liner in a cereal box, it is polypropylene. This keeps your cereal dry and fresh. PP is also commonly used for disposable diapers, pails, plastic bottle tops, margarine and yogurt containers, potato chip bags, straws, packing tape and rope.
Only about 3% of PP products are currently being recycled in the US, check your local recycling programs to see if they accept PP. Recycled PP is used to make landscaping border stripping, battery cases, brooms, bins and trays.
PP #5 is considered safe for reuse.
- #6 PS (Polystyrene)
-
Polystyrene is an inexpensive, lightweight and easily-formed plastic with a wide variety of uses. It is most often used to make disposable styrofoam drinking cups, take-out "clamshell" food containers, egg cartons, plastic picnic cutlery, foam packaging and those ubiquitous "peanut" foam chips used to fill shipping boxes to protect the contents. Polystyrene is also widely used to make rigid foam insulation and underlay sheeting for laminate flooring used in home construction.
Because polystyrene is structurally weak and ultra-lightweight, it breaks up easily and is dispersed readily throughout the natural environment. Beaches all over the world have bits of polystyrene lapping at the shores, and an untold number of marine species have ingested this plastic with immeasurable consequences to their health.
Polystyrene may leach styrene, a possible human carcinogen, into food products (especially when heated in a microwave). Chemicals present in polystyrene have been linked with human health and reproductive system dysfunction.
Recycling is not widely available for polystyrene products. Most curb side collection services will not accept polystyrene, which is why this material accounts for about 35% of US landfill material. While the technology for recycling polystyrene is available, the market for recycling is small. Awareness among consumers has grown, however, and polystyrene is being reused more often. While it is difficult to find a recycler for PS, some businesses like Mailboxes Etc. which provide shipping services are happy to receive foam packing chips for reuse.
Polystyrene should be avoided.
- #7 Other (BPA, Polycarbonate and LEXAN) plastic baby bottles
-
The #7 category was designed as a catch-all for polycarbonate (PC) and "other" plastics, so reuse and recycling protocols are not standardized within this category. Of primary concern with #7 plastics, however, is the potential for chemical leaching into food or drink products packaged in polycarbonate containers made using BPA (Bisphenol A). BPA is a xenoestrogen, a known endocrine disrupter.
Number 7 plastics are used to make baby bottles, sippy cups, water cooler bottles and car parts. BPA is found in polycarbonate plastic food containers often marked on the bottom with the letters "PC" by the recycling label #7. Some polycarbonate water bottles are marketed as non-leaching for minimizing plastic taste or odor, however there is still a possibility that trace amounts of BPA will migrate from these containers, particularly if used to heat liquids.
A new generation of compostable plastics, made from bio-based polymers like corn starch, is being developed to replace polycarbonates. These are also included in category #7, which can be confusing to the consumer. These compostable plastics have the initials "PLA" on the bottom near the recycling symbol. Some may also say "Compostable."
#7 plastics are not for reuse, unless they have the PLA compostable coding. When possible it is best to avoid #7 plastics, especially for children's food. Plastics with the recycling labels #1, #2 and #4 on the bottom are safer choices and do not contain BPA. PLA coded plastics should be thrown in the compost and not the recycle bin since PLA compostable plastics are not recyclable.
When the well's dry, we know the worth of water. Benjamin Franklin
Tap Water
Is your tap water is safe to drink? According to the Environmental Working Group, testing by water utilities has found more than 300 pollutants in the tap water Americans drink. More than half of the chemicals detected are not subject to health or safety regulations and can legally be present in any amount. The federal government (EPA) does have health guidelines for others, but 49 of these contaminants have been found in one place or another at levels above those guidelines, polluting the tap water for 53.6 million Americans. Water utilities spend 19 times more on water treatment chemicals every year than the federal government invests in protecting lakes and rivers from pollution in the first place.
EWG's Tap Water Database
With this tool, you enter your zip code to get a FREE report on the contaminants that flow from your tap. More than 4 million Americans live in places where contaminants in drinking water exceed a legal limit. Poor, rural areas are often more affected than wealthy, urban and suburban ones. EWG's Tap Water Database pulls about 30 million records, mostly from state agencies.
Comparison of Bottled Water with Municipal (Tap) Water
Source: Dr. Mary Shackelton, MPH, ND

Learn about the problems with bottled water, and why drinking tap water is a better alternative.
- City tap water cannot have E. coli or fecal coliform bacteria. FDA bottled water rules include no such prohibition (a certain amount of any type of coliform bacteria is allowed in bottled water).
- Most cities using surface water have had to test for Cryptosporidium or Giardia, two common water pathogens that can cause diarrhea and other intestinal problems, yet bottled water companies have no such test.
- City tap water must meet standards for certain important toxic or cancer-causing chemicals, such as phthalate (a chemical that can leach from plastic, including plastic bottles); some in the industry persuaded FDA to exempt bottled water from the regulations regarding these chemicals.
- City water systems must issue annual "right to know" reports, telling consumers what is in their water. Bottlers successfully killed a "right to know" requirement for bottled water.
Common sources of drinking water contamination.
- Naturally Occurring Contamination Sources
- Microorganisms (wildlife and soils), radionuclides (underlying rock), nitrates and nitrites (nitrogen compounds in the soil), heavy metals (underground rocks containing arsenic, cadmium, chromium, lead, and selenium), fluoride.
- Contamination Resulting from Human Activities
- Bacteria and nitrates (human and animal wastes - septic tanks and large farms), heavy metals (mining, construction, fruit orchards), fertilizers and pesticides (anywhere crops or lawns are maintained), industrial products and wastes (factories, industrial plants, gas stations, dry cleaners, leaking underground storage tanks, landfills, and waste dumps), household wastes (cleaning solvents, used motor oil, paint, paint thinner), lead and copper (household plumbing materials), water treatment chemicals (wastewater treatment plants) pharmaceutical drugs (many drug components are not removed from wastewater treatment processes and are returned to our lakes and rivers).
Unlike pollution from a specific location, or "point," such as industrial and sewage treatment plants, non-point source pollution comes from many diffuse sources. Non-point pollution is caused by rainfall or snowmelt moving over and through the ground. As the runoff moves, it picks up and carries away natural and human-made pollutants, finally depositing them into lakes, rivers, wetlands, coastal waters and ground waters. Non-point source pollution can include:
- Runoff fertilizers, herbicides, fungicides and insecticides. Commercial agriculture isn't the only source of these toxins, every year homeowners apply 67 million pounds of chemical fertilizer, pesticides, fungicides and herbicides to lawns and gardens.
- Oil, grease and toxic chemicals from urban runoff and energy production.
- Sediment from construction sites, crop and forest lands, and eroding coastlines and stream banks.
- Salt from irrigation practices.
- Acid drainage and toxic chemicals and metals from mining.
- Bacteria and nutrients from livestock, pet wastes and faulty septic systems.
- Atmospheric deposition and hydro modification.
- We produce more than 230 million tons of municipal solid water that contain bacteria, nitrates, viruses, synthetic detergents, and household chemicals (approx. 5 pounds of garbage per person daily).
- More than 12 million recreational boats and 10,000 boat marinas release solvents, gasoline, detergents, and raw sewage directly into our rivers, lakes and streams annually.
- Worldwatch Institute estimates that in the United States, 18,000 golf courses cover more than 1.7 million acres and soak up nearly 4 billion gallons of water daily. They also use massive amounts of pesticides, herbicides and fertilizers that contribute to the pollution of our drinking water.
Public and community water utilities use a variety of treatment processes in the escalating toxicity war to try to remove contaminants from our drinking water. Commonly used large-scale decontamination processes include; coagulation (flocculation and sedimentation), filtration and chemical disinfection. Ion exchange is sometime used to soften water, deionize it, and is used in desalination. Adsorption is sometimes used (adsorption is the adhesion of atoms, ions, or molecules from contaminated water solid to a surface).
- Coagulation: removes dirt and other particles suspended in water. Alum and other chemicals are added to water to form tiny sticky particles called "floc" which attract the dirt particles. The combined weight of the dirt and the alum (floc) become heavy enough to sink to the bottom during sedimentation.
- Sedimentation: The heavy particles (floc) settle to the bottom and the clear water moves to filtration.
- Disinfection: Chlorine, or another disinfection method is used to kill bacteria or microorganisms.
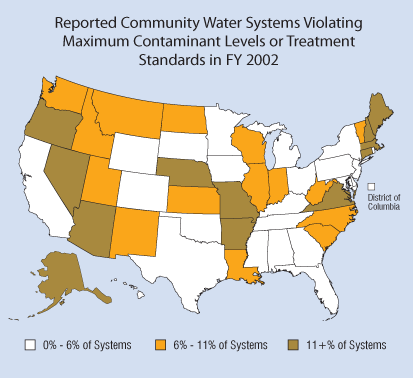
In addition to the problem of of community water utilities violating maximum contaminant levels, in 2001, 25% of community water utilities failed to conduct the testing, or report the results, required to verify that their drinking water was safe. (Graphic source: EPA)
According to the EPA: Under the Safe Drinking Water Act (SDWA), EPA sets legal limits on the levels of certain contaminants in drinking water. The legal limits reflect both the level that protects human health and the level that water systems can achieve using the best available technology, and taking cost into consideration. EPA regulations are based on a human health criterion, which is the highest concentration of a pollutant in water that is not expected to pose a significant risk to human health.
EPA analytical methods for determining drinking water safety come from a variety of sources. Many are developed by EPA or other governmental organizations. Consensus method organizations such as Standard Methods and ASTM, International often provide methods suitable for drinking water analyses. Methods may be developed by university, commercial or public water system laboratories, and by commercial vendors.
EPA "acceptable levels" are determined using maximum-levels criterion:
- Maximum Contaminant Level Goal (MCLG): The level of a contaminant in drinking water below which there is no known or expected risk to health. MCLGs allow for a margin of safety and are non-enforceable public health goals.
- Maximum Contaminant Level (MCL): The highest level of a contaminant that is allowed in drinking water. MCLs are set as close to MCLGs as feasible using the best available treatment technology and taking cost into consideration. MCLs are enforceable standards.
- Maximum Residual Disinfectant Level Goal (MRDLG): The level of a drinking water disinfectant below which there is no known or expected risk to health.
- Maximum Residual Disinfectant Level (MRDL): The highest level of a disinfectant allowed in drinking water.
Besides prescribing maximum legal limits for health threatening contaminants, EPA rules set water-testing schedules and methods that water utilities must follow. The rules also list acceptable techniques for treating contaminated water. SDWA gives individual states the opportunity to set and enforce their own drinking water standards if the standards are at least as strong as EPA's national standards.
The EPA has an impossibly difficult job, and many employees are trying their best. In an organization as titanic as the federal government, decision making, policy, and law making are influenced by many competing, and powerful, interests. Your health might not be their first priority.
 Drinking Water Standards and Health Advisories
Drinking Water Standards and Health Advisories-
The guidelines in this booklet provide RfD (reference dose) and Cancer values for the listed chemicals and micro-bacteriogical contaminants found in drinking water. The list is long but far from all inclusive. Approximately 3,000 new chemicals are introduced into the environment every year. This is an overwhelming amount for any regulatory body, even an agency as large as the EPA.
-
 Water on Tap: what you need to know
Water on Tap: what you need to know -
Water on Tap is a consumer-oriented booklet filled with interesting graphics, facts and informative tables. It answers the questions; Where does your drinking water come from? How do you know if your drinking water is safe? How can you protect it? What can you do if there's a problem with your drinking water? The National Primary Drinking Water Standards tables at the end list the specific chemicals EPA recommends testing for, their effects and common sources.
How to Make Your Drinking Water Safe
-
The first step is to have your water tested. It is impossible to determine whether water is safe to drink by appearance or odor. Simple procedures such as boiling or the use of a household activated carbon filter are inadequate for eliminating all possible contamination. Even "natural spring water" must be tested to determine if decontamination treatment is required. Laboratory chemical and microbiological analysis is expensive, but still the best way to help you decide on the appropriate method of purification for your situation. If you are on a corrective hTMA protocol, you may want to have your water tested for specific minerals that may contribute, or hinder, the success of therapy.
Water Testing Options
- Professional Services
- National Testing Laboratories
- Drinking Water Specialists
- Home Water Purification Systems
- Ozark Water Service and Air Services
- Hague Quality Water International
- Good Water Company
- DIY Water Test Kits
- Industrial Test Systems, Inc.
- American Water Service PurTest® home drinking water test kits
- Baldwin Meadows 14-in-1 Drinking Water Test Kit Well Water and Tap Water
- The second step is to install an appropriate water purification system. Deciding which purification (filtration) method to choose depends on your water test results (quality) and your budget. It can be complicated, some people work with a water purification professional (see resources below). Essentially, you filter your water to reduce concentrations of particulate matter, including suspended particles, parasites, bacteria, algae, viruses, fungi. Also, it is necessary to remove an increasing number of chemicals and dissolved or particulate materials that come from toxic surfaces that rainwater may have come into contact with. Do not forget to evaluate your water delivery system (i.e., plumbing and the well system if you have a well). Also, ensure that you use a drinking water safe water hose if you use one to drink from, or to fill water containers for animals. Most water hoses are manufactured using known carcinogens which can leach into the water as it passes through.
Demineralized Water
Water purification involves processing water to remove unwanted contaminants (also referred to as demineralization). Water demineralization removes health supporting minerals that are found naturally in water too. Most water re mineralization efforts are done strictly for commercial or industrial purposes. A World Health Organization report, ‘Health Risks From Drinking Demineralized Water,’ discusses that consumption of demineralized water (distilled, reverse osmosis, deionized, desalinated) poses several health risks that may include damage to intestinal mucosa and loss of minerals due to increased diuresis. The report states that chemically pure water (e.g., distilled, reverse osmosis, de-ionization) should be re mineralized before consumption, and that it is difficult, if not impossible, to reconstruct the complexity of the structure found in natural water. Avoiding common table salt and increasing your use of high-quality sea salt can help. The safest, most effective way to ensure you have the proper mineral levels and ratios to support healthy cellular function is with hTMA guided nutrition.
Possibly none of the commonly used ways of re-mineralization could be considered optimum since the water does not contain all of its beneficial components. Current methods of stabilization are primarily intended to decrease the corrosive effects of demineralized water. Health risks from drinking demineralized water
Commercially and municipally demineralized water is commonly "stabilized" by adding minerals and trace elements after processing. The purpose for this is not to replace essential nutrients, it is to make water taste or smell better, or make it more stable or less corrosive for use in various industrial processes. If improved health was the reason for re mineralization, the choices and forms of minerals (and their ratios) would be very different. Households who use demineralization on their drinking water to purify it seldom, if ever, do anything to remineralize it, so many people are now drinking demineralized water.
Demineralized water has little or no minerals. If you are using a distiller, a reverse osmosis unit (RO), or de-ionization (ion exchange resins) you are demineralizing your water. Research on heart disease and cancer has demonstrated that hard water and water that is moderately high in TDS (total dissolved solids) has a health benefit. Purifying devices remove everything from your water, not only harmful bacteria and toxic minerals, but beneficial minerals as well. This nutrient-mineral stripped water cannot sustain life even in a fish bowl. Fish require minerals, and if placed in demineralized water they perish.
Any time a hypotonic (de-mineralized) solution comes into contact with a hypertonic (mineralized) solution, the minerals within the hypertonic solution will transfer out and into the hypotonic solution until equilibrium is achieved. When one drinks hypotonic water, the minerals in the blood and lymphatic system, which are hypertonic, transfer into the hypotonic reverse-osmosis or distilled water that is consumed and the minerals can then be flushed out of the body (urination).
In a desperate effort to re-mineralize, the blood and lymphatic systems begin to scavenge for minerals from other parts of the body, such as bones and other organs, and this process repeats itself every time de-mineralized hypotonic water is re-consumed. Several studies suggest that people who drink de-mineralized water (hypotonic) over a long period of time tend to be more prone to degenerative diseases such as osteoporosis.
Simply put, if demineralized water is ingested for long periods of time, it will leach essential nutrient minerals such as potassium, magnesium, sodium, calcium and others. Mineralized water supports immeasurable cellular functions, and when there are no minerals in your drinking water, minerals will be taken from somewhere in your body to satisfy its functional requirements.
Demineralized water is not considered ideal drinking water. Consumption may not provide adequate levels of beneficial nutrients. Sufficient evidence is now available to confirm the health consequences from drinking water deficient in calcium or magnesium. Drinking water should contain minimum levels of certain essential minerals. F. Kozisek
How to Remineralize Water
Minerals and trace minerals are essential catalysts for all biological processes that develop and maintain good health. If your drinking water is demineralized, you can remineralize it by adding trace minerals. Remineralizing also will improve the flavor of distilled, reverse osmosis or purified water. You can use a product such as ConcenTrace® Trace Mineral Drops to remineralize your drinking water. Remineralization compares to mineral waters and adds a complete, balanced spectrum of low sodium minerals and trace minerals. Another product, Sea-Crop® may be used as a mineral supplement for animal nutrition.
- ConcenTrace® Trace Mineral Drops Remineralizing drinking water with 20-40 drops per gallon or 2-4 drops per glass, adds a complete balanced spectrum of low sodium minerals and trace minerals.
- Sea-Crop® Sea-Crop is a concentrate that contains all of the minerals and trace minerals found in seawater in concentrated form, but with the sodium chloride 95% reduced.
Recommended Drinking Water
The best water to drink is clean and contains naturally occurring minerals. Natural water, is a type of "whole-food." It hydrates the body, flushes toxins from the body and supplies many needed minerals in a bioavailable form. Processed water is tampered with by adding things to it, filtering it, heating it, alkalinizing it, etc., until it no longer provides the chemical components (in the correct amounts and ratios). Our cells have evolved to rely on the chemical composition of natural water for all their life supporting functions.
- Natural spring and artesian water
- Natural spring and artesian water flows up from a natural spring and is bottled at the source. There are several issues to consider concerning bottled water. A quality spring water hydrates the body well and contains a variety of essential trace minerals. Unfortunately even natural water sources can become contaminated and must be tested to ensure purity and quality.
- Natural mineral water
- Mineral water could come from a natural spring or an artesian well. Basically it comes from an underground source and contains at least 250 parts per million (ppm) of dissolved solids, including nutrient minerals and trace elements. Again, natural water sources can become contaminated and must be tested.
- Well water
- Well water comes from a hole drilled in the ground that taps into an underground water source. A pump then brings the water to the surface. Well water is commonly contaminated. If you are drinking well water you need to have your water thoroughly tested. Inexpensive municipal water tests are not comprehensive, they typically test only for coliform bacteria, nitrates, total dissolved solids, and pH levels. Most heavy metals, minerals and dangerous chemicals are not tested for. Pharmaceutical contaminants, for example, are not filtered out by municipal water treatment plants. You need to get a test that's thorough enough to determine the purification system that is appropriate for decontaminating your specific drinking water source.
Water Filtration
Most of us need to filter the water we drink. There is no perfect water processing solution, because any processing denatures water. Like so many purchases we make, choosing the right water filter/system is overly complicated, even though they all rely on just a few basic technologies to remove contaminants. Not every product is as good as another. Be sure that the product you choose adequately addresses your specific need. Proper maintenance is critical also.
Some products or systems use a combination of filter technologies, while others rely on a single technology. To ensure that a filter removes a particular contaminant, verify that it is properly certified to remove that specific contaminant. For instance, one brand might remove chloramine but another brand can't even though they are both carbon filters. Remember, water filters vary greatly in quality. Some filters are labeled ‘NSF certified’ but are not all NSF certifications are the same. A filter may be "certified" that it will improve taste and smell but not that it will remove a specific contaminant.
Occasionally, there may be benefits to drinking demineralized (purified) water for chelation for short periods of time. Using demineralized water can be dangerous because of the rapid loss of electrolytes (sodium, potassium, chloride) and trace minerals. Loss of magnesium can cause heart beat irregularities and high blood pressure. Multi-stage water filters may be needed depending on the impurities in your household water. If you demineralize your household water, you need to find a good source of natural water to use for drinking and cooking that contains essential nutrient minerals.
Ingestion of harmful chemicals from drinking water is not the only route of exposure. Both absorption through the skin and inhalation have been studied. For example, absorption rates for the toxic chemicals toluene, ethyl benzene and styrene show significantly higher rates of absorption from skin than through oral ingestion. TCE (trichloroethylene) absorption from taking a shower was 6 to 80 times greater than from drinking water. Consider a using point-of-use shower filter for chemical removal.
Public swimming pools and hot tubs are highly toxic and should be avoided. While swimming is an excellent no-impact exercise and recreation, swimming in a chlorinated swimming pool puts you at risk from disinfection byproducts (DBPs). DBPs are formed when organic materials (e.g. hair, skin, sweat, dirt and urine etc.) react with the chlorine used for sanitization. DBPs are over 1,000 times more toxic than chlorine.
Back to TopWater Filtering Methods and Technologies
- Community Tap Water
-
 Before any post-purification that's done in the home, the municipal supplied water from your faucet has been filtered, treated, processed and disinfected at the water utility facility. It is chemically treated with chlorine (and other chemicals), and fluoride is commonly added. Chlorine is linked to cancer.
Before any post-purification that's done in the home, the municipal supplied water from your faucet has been filtered, treated, processed and disinfected at the water utility facility. It is chemically treated with chlorine (and other chemicals), and fluoride is commonly added. Chlorine is linked to cancer.Fluoride in drinking water has been proven to have no benefits and a multitude of harmful health effects. Less well known, but perhaps more serious, is the fact that many chemicals and pharmaceutical drugs are not removed by municipal water cleaning processes, so they are reintroduced into the water supply (thus, multiplying concentrations over time). Toxic metals that are present in municipal tap water supplies include aluminum, copper, bromine and others.
Fluoride causes more cancer death, and causes it faster than any other chemical. Dr. Dean Burk, National Cancer Institute
- Distillation Method
- Distilled water is created by heating water to it's steam state, separating it from solid residues (e.g., minerals), then it is collected as condensation. Distillation removes some bacteria, viruses, and chemicals with a boiling point that is higher than water's boiling point. Distillation does not remove chemicals such as chlorine, trihalomethanes, volatile organic chemicals (VOCs) or pharmaceutical compounds. Distilled water has no minerals and thus acts as a chelator by attracting minerals it comes in contact with in the body. Distilled water may be used for drinking occasionally but is not recommended for drinking for any length of time because of its chelation effect. Long term use is harmful because it will deplete the body of needed essential nutrient minerals. Under the guidance of a qualified health practitioner, distilled water may be useful for short periods of time for aiding in detoxification.
- Carbon Filtering Material
- There are many, many types of carbon-based filter products on the market, making choosing this type of filter a confusing challenge because they vary significantly in effectiveness, quality, and cost. Carbon and activated carbon filters remove contaminants from water by chemically bonding with them. Some remove chlorine and improve taste and odor only. Others can remove a range of contaminants including asbestos, lead, mercury, and volatile organic compounds (VOCs). Activated carbon material cannot effectively remove common pollutants such as arsenic, fluoride, hexavalent chromium, nitrate and perchlorate. Generally, carbon filters come in two forms, carbon block and granulated activated carbon.
- Carbon Block filters use pulverized activated carbon that's been molded into blocks with high pressure. They are commonly more effective than granulated activated carbon filters due to larger surface area. Efficacy also is determined by the rate at which water flows through the block.
- Granulated Activated Carbon filters contain fine grains of activated carbon. They are typically less effective than carbon block because they have an overall smaller surface area. Like carbon block, efficacy also is determined by the water flow-rate.
- Ceramic Filtering Material
- Effectiveness of ceramic filters at removing bacteria, viruses, and protozoa depends on the production quality of the ceramic filter. Most ceramic filters are effective at removing bacteria and the larger protozoans, but not at removing the viruses. Studies have shown adequate removal of bacterial pathogens in water filtered through high quality locally produced or imported ceramic filters in developing countries. A 60-70% reduction in diarrheal disease incidence has been documented in users of these filters. Studies have also shown significant bacterial contamination when poor-quality locally produced filters are used, or when the receptacle is contaminated at the household level. Therefore, it is important that users be trained to properly care for and maintain the ceramic filter and receptacle. Currently, the most widely implemented ceramic filter is the Potters for Peace design.
- Reverse Osmosis Method
- Reverse osmosis water has been forced through membranes that remove larger particles, pollutants and minerals. Reverse osmosis can make your water more acidic. Purified water is an active absorber, and when it comes into contact with air, it absorbs carbon dioxide, making it acidic. The more purified water a person drinks, the higher the body acidity becomes. Reverse osmosis water is a mineral-deficient water and will remove minerals from the body, does not hydrate the body well and always contains residues of the plastic polycarbonate or other membrane the water passes through. If filters are not changed frequently, reverse osmosis systems can harbor many bacteria and viruses.
- Water Deionization Technology
- Deionized water has had ionized impurities and minerals removed from it. DI water is water that has the ions removed. In contrast, tap water usually has ions from the soil (Na+, Ca2+), from the pipes (Fe2+, Cu2+), and other sources. Water is usually deionized by using an ion exchange process. Deionized water isn't necessarily pure water, given the usual de-ionization procedure. Non-ionic contaminants may persist. For example, bacteria and pathogens are not removed by deionizing water. These filters remove mineral salts and other electrically charged molecules (ions) from water. The process cannot remove non-ionic contaminants (including trihalomethanes and other common volatile organic compounds) or microorganisms.
- UV (ultraviolet) Water Disinfectant Technology
- Ultraviolet systems use light frequency to kill bacteria and other microorganisms. UV treatment does not remove chemical contaminants. UV radiation can be an effective viricide and bactericide. Disinfection using UV radiation is commonly used in wastewater treatment applications and is finding an increased usage in drinking water treatment. Many bottlers of spring water use UV disinfection equipment to sterilize their water. Solar water disinfection is the process of using PET bottles and sunlight to disinfect water. Ultraviolet germicidal irradiation is the generic process to inactivate microorganisms in water, air, medical environments, etc.
Water Softeners
Water softeners do not purify water. Drinking softened water has been shown to increase heart disease. Water softening systems treat hard water using an ion-exchange. A cylinder inside a water softener contains sodium chloride or potassium chloride. These chlorides attract the calcium and magnesium which render the water softer. Soft water has a low amount of minerals. However, sodium or potassium chloride is also released into the water during this process. Water softeners regenerate by flushing the buildup of calcium and magnesium in the brine in the cylinder. During the regeneration cycle the sodium or potassium saturated water is flushed into the sewer system. This is causing environmental problems. Sodium and potassium saturated water harms fish and aquatic life, impacts crops irrigated with water, and affects downstream users.
Water Information Sources
- National Ground Water Association (NGWA)
- NGWA is a trade association for ground water professionals including well drillers, pump installers, geologist and other scientists. It is the NGWA vision to promote the responsible development, use, and management of ground water resources. ngwa.org The NGWA has developed a site devoted to informing consumers about ground water and water wells. This site provides useful information about well maintenance, water quality, ground water and well basics. You can also use this site to locate a contractor in your area. wellowner.org
- The Water Quality Association (WQA)
- WQA is a not-for-profit international trade association representing the residential, commercial, industrial, and small community water treatment industry. WQA maintains a close dialogue with other organizations representing different aspects of the water industry in order to best serve consumers, government officials, and industry members. They offer certification to members based upon required attendance to educational classes and testing. The website lets homeowners find professionals in their area. wqa.org
- The Water Systems Council (WSC)
- WSC is the only national, non-profit organization solely focused on household wells and small water well systems. Committed to ensuring that Americans who get their water from household, private wells have safe reliable drinking water. Water Systems Council
- The American Ground Water Trust
- AGWT is a not-for-profit educational organization dedicated to promoting efficient and effective ground water management. This website provides educational information about ground water issues that are important to water well owners. agwt.org
- The Environmental Protection Agency (EPA)
- EPA is the federal agency, whose function is to protect the environment. This includes the protection of public water supplies and our natural water ways. The site below gives information on the regulations that pertain to public water supplies, including what they need to test for and the levels which are considered safe. epa.gov/drink The United States Geological Survey (USGS) is a not-for-profit educational organization dedicated to promoting efficient and effective ground water management. This website provides educational information about ground water issues that are important to water well owners. water.usgs.gov
- Alternatives for Managing the Nation's Complex Contaminated Groundwater Sites
-
National Academy of Sciences - special report
At hundreds of thousands of hazardous waste sites (300,000+) across the country, groundwater contamination remains at levels that exceed cleanup goals. The most problematic sites are those with potentially persistent contaminants including chlorinated solvents recalcitrant to biodegradation, and with hydrogeologic conditions characterized by large spatial heterogeneity or the presence of fractures. The majority of sites that have been decommissioned were relatively simple to clean up compared with the remaining hazardous waste sites. In 2004, the U.S. Environmental Protection Agency estimated that more than $209 billion would be needed to mitigate these hazards over the next 30 years (likely an underestimate because this number did not include sites where remediation was already underway or where remediation had transitioned to long-term management).
The Department of Defense (DoD) has made substantial financial investments (over $30 billion) to address legacy cleanup responsibilities of some of their hazardous waste sites. Although many hazardous waste sites at military facilities have been closed, meeting goals such as safe drinking water standards in contaminated groundwater has rarely occurred at many complex DoD sites. It is probable that these sites will require significantly longer remediation times than originally predicted and, continued increasing financial demands for monitoring, maintenance, and reporting.
This report assesses the future of the nation's groundwater remediation efforts focusing on the technical, economic, and institutional challenges facing the Army and other responsible parties as they pursue site closure. Previous NRC reports concluded that complete restoration of contaminated groundwater is unlikely to be achieved for many decades for a substantial number of sites, in spite of the fact that technologies for removing contaminants from groundwater have continued to evolve and improve.
Download: Alternatives for Managing the Nation's Complex Contaminated Groundwater Sites
Resources
- Video
-
- Water fluoridation officially linked to cognitive deficits.
- Moms Against Fluoridation
- 10 Facts About Fluoride Fluoride Action Network (fluoridealert.org)
- Fluoride: Poison On Tap (1hr 30min)
- Do not swallow fluoride (51 sec.) from Fluoride: The Hard to Swallow Truth Documentary
- Our Daily Dose (20 min) Science regarding the safety of fluoride.
- The Fourth Phase of Water Professor Gerald Pollack
- The Story of Bottled Water (short, 8 min.)
- Water: The Great Mystery (Documentary film)
- An Inconvenient Tooth
- Flow: For the Love of Water (Documentary film)
- Tapped (Documentary film)
Back to Top
- Books / PDF
-
- Water and its Impact on Health David L. Watts, Ph.D.
- 10 Facts About Fluoride Fluoride Action Network
- Lawsuit to End Water Fluoridation in the US
- Safe Drinking Water Guide Environmental Working Group
- Living Rainbow H2O Mae-Wan Ho
- Don't Drink The Water Guide to Contaminated Drinking Water
- The Drinking Water Book How to Eliminate Harmful Toxins from Your Water
- Blue Revolution: Unmaking America's Water Crisis Cynthia Barnett
- Rain: A Natural and Cultural History Cynthia Barnett
Back to Top
- Websites / Articles
-
- Find-a-Spring
- Pollack Laboratory Research Themes:
- Food & Water Watch
- Nutrients in drinking-water World Health Organization
- Drinking Water Information
- Tap water info Environmental Working Group
- Endocrine Disrupting Chemicals in Drinking Water testimony before a congressional committee
- Colorado streams and ground water contain wastewater and chemicals
- Emerging Chemicals of Concern California Department of Toxic Substances Control
- Pharmaceutical drugs in wastewater and drinking water
- Illicit Drugs in Drinking Water The Institute of Science in Society
- Industrial Agriculture: Water in farm country dangerously polluted
- Water testing can determine if your household water is safe
- Fluoride information
- European Hydration Institute (Children's needs)
- World Water Day
- Water info from U.S. Environmental Protection Agency (EPA)
- National Sanitation Foundation
- Water in America: Is It Safe to Drink? National Geographic
- New Study Links Water Fluoridation To Hypothyroidism
- Water, Water Everywhere…and Not a Drop to Drink Dr. Mary Shackelton, MPH, ND
- Latest Message from Water: Is Dr. Emoto a Spiritual Madoff?
- Dangers of plastic water bottles Plastic By The Numbers - Eartheasy.com
- What You Need To Check Next Time You Buy Bottled Water Healthy Food House.com
Back to Top
- Products
-
- Water Distillers (steam distillation combined with carbon post-filtration) Waterwise water distillers are engineered around the proven steam distillation process. Combined with carbon filtration, distillation is the water treatment technology that most completely reduces the widest range of contaminants, including biological, organic and inorganic elements. In fact, a quality distillation system provides water that is up to 99% free of impurities, including heavy metals, chemicals and microorganisms.
- Berkey Water Filter Berkey water filter systems remove harmful pathogenic bacteria, cysts, parasites, and unhealthy chemical contaminants such as Chlorine to levels higher than 99.99%, while at the same time leaving in the essential minerals your body needs.
- ConcenTrace® Trace Mineral Drops Remineralizing drinking water with 20-40 drops per gallon or 2-4 drops per glass, adds a complete balanced spectrum of low sodium minerals and trace minerals.
- Sea-Crop® Sea-Crop is a concentrate that contains all of the minerals and trace minerals found in seawater in concentrated form, but with the sodium chloride 95% reduced.
- LifeStraw® Water Filter Note: This product does noes not remove chemicals. LifeStraw water filter products provide safe drinking water by filtering microbiologically contaminated water (only). All LifeStraw products are durable, lightweight, and require no electricity or batteries to operate.
- Water Hose (drinking water safe) Unfortunately, most water hoses are manufactured using known carcinogens which can leach into the water as it passes through. This is an important consideration if you fill your animal's waterers with a hose. Or, if you water your garden vegetables with it.